- Knowledge Base
- Workflow
- Here
How should I adjust my hybridization program for an AT-Rich panel with Trinity Freestyle™?
Introduction
As an expansion of the Trinity™ sequencing product line, Trinity Freestyle delivers the same workflow and performance improvements to traditional hybrid capture workflows, and is additionally compatible with libraries generated with the Twist FlexPrep and EF 2.0 kits.
As with any hybrid capture workflow, the hybridization program can be adjusted to maximize uniformity and specificity in a way that best suits your panel design. Here we demonstrate the impact of modifying hybridization temperature for an AT-rich panel using the Twist Genotyping Panel–Human 600K and the Trinity Freestyle workflow.
Why Modify Hybridization Temperature?
Hybridization temperature can be adjusted to tune panel performance. For AT-rich panels, probes will bind to target sequences at lower temperatures than for GC-rich panels. While higher hybridization temperatures can dissociate off-target reads and increase on-target rate, they can negatively affect binding to AT-rich regions, coming at the cost of AT-dropout and reduced uniformity. A lower hybridization temperature can improve the binding of AT-rich molecules, decreasing AT-dropout and improving uniformity for AT-rich panels, but lower temperatures can also increase off-target binding, reducing specificity.
With the Trinity Freestyle workflow, we demonstrate a modification to the hybridization program that provides the best of both: a lower hybridization temperature improves the capture of AT-rich molecules, while a temperature ramp-up at the end of the program dissociates off-target reads (without affecting the already-hybridized AT-rich reads) to maintain a high on-target rate and good uniformity.
Demonstration with the Genotyping Panel–Human 600K
We tested the effects of four different hybridization programs using the AT-rich Genotyping Panel–Human 600K (Twist Bioscience). All tests were run with the same set of libraries, which were prepared with HG001 human genomic DNA and the Twist FlexPrep UHT Library Prep & Fast Hyb with Trinity Freestyle protocol and reagents. Hybridization input was adjusted to suit the panel size.
The 1-hour hybridization program in the Trinity Freestyle protocol was modified to test the following temperatures: 70°C, 65°C, 62°C, plus a temperature ramp-up condition which involved a 1-hour hybridization at 60°C followed by 5 minutes at 65°C and 5 minutes at 70°C (Table 1). After hybridization, all reactions proceeded with no changes to the Trinity Freestyle protocol, and reactions were sequenced on AVITI with a Trinity Freestyle 2x75 Sequencing Kit. Data was downsampled to ~5Gb/sample after sequencing for secondary analysis.
|
Step |
Temperature (°C) |
Duration |
|
Initialization |
95°C |
5 minutes |
|
Hybridization |
60°C |
1 hour |
|
Increase 1 |
65°C |
5 minutes |
|
Increase 2 |
70°C |
5 minutes |
Table 1. Thermal cycling parameters for 1-hour hybridization reaction with temperature ramp-up for AT-rich panels. Lid temperature should be set to 85°C.
Temperature has a strong influence on secondary metrics such as AT-dropout, fold-80, and on-target rate (Figure 1). As expected, the 70°C hybridization maintains a high on-target rate, but fold-80 suffers due to high AT-dropout. In contrast, lowering the hybridization temperature to 65°C or 62°C improves AT-dropout and fold-80, but reduces on-target rate. This is likely due to off-target binding at these lower temperatures.
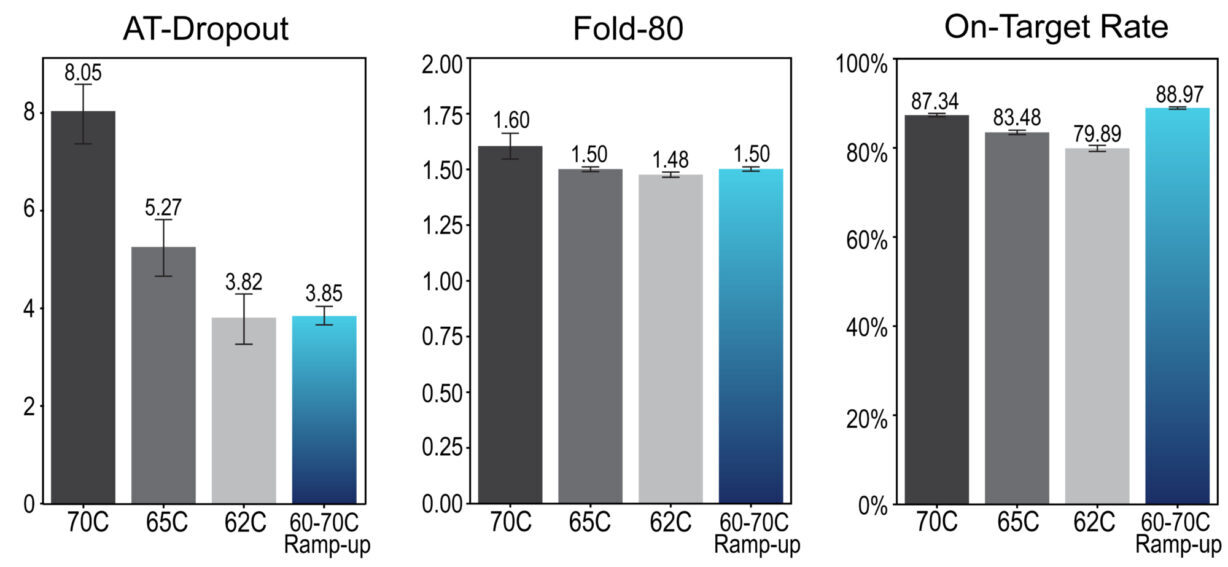
Figure 1. AT-dropout, Fold-80, and on-target rate across four different hybridization programs. Libraries were prepared and sequenced with Trinity Freestyle.
The 60°C hybridization with ramp-up to 70°C (shown in Table 1) reduces AT-dropout and keeps fold-80 low, without compromising on-target rate (Figure 1). Compared to the other hybridization conditions which all require a trade-off between specificity and uniformity, this condition optimizes both, providing the highest on-target rate and lowest fold-80. Primary metrics remain within their expected ranges regardless of the hybridization program used (Figure 2).
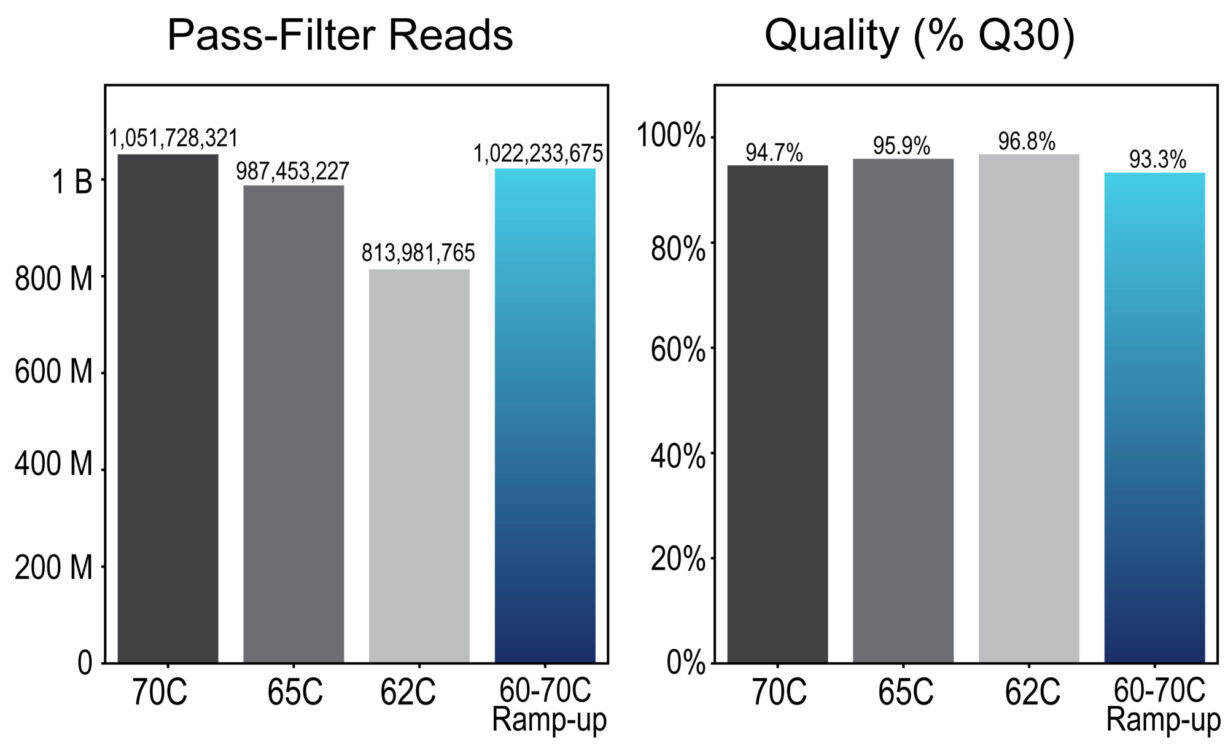
Figure 2. Pass-filter reads and quality (% Q30) across four different hybridization programs. Libraries were prepared and sequenced with Trinity Freestyle.
Recommendations and Application to Other Panels
For the Twist Genotyping Panel–Human 600K, we recommend adjusting the hybridization step to use the temperature ramp-up program shown in Table 1. This adjustment to the hybridization program maximizes both specificity and uniformity metrics.
While the results above focus on a single AT-rich panel, this hybridization program may also improve performance for other AT-rich panels sequenced with Trinity Freestyle or Trinity. For those troubleshooting high AT-dropout in their Trinity Freestyle or Trinity libraries, you may also find that this program improves your results as well.
More details about sequencing AT-rich panels with Trinity Freestyle can be found in the Trinity Workflow for AT-rich Panels Technical Note.
For more information, reach out to your Field Applications Scientist or Element Biosciences Support at support@elembio.com.
Related Articles
We’re here to help — if you can’t find what you’re looking for, let us know.